Health
Dostarlimab, The Drug That Cured All Cancer Patients For ‘First Time In History’
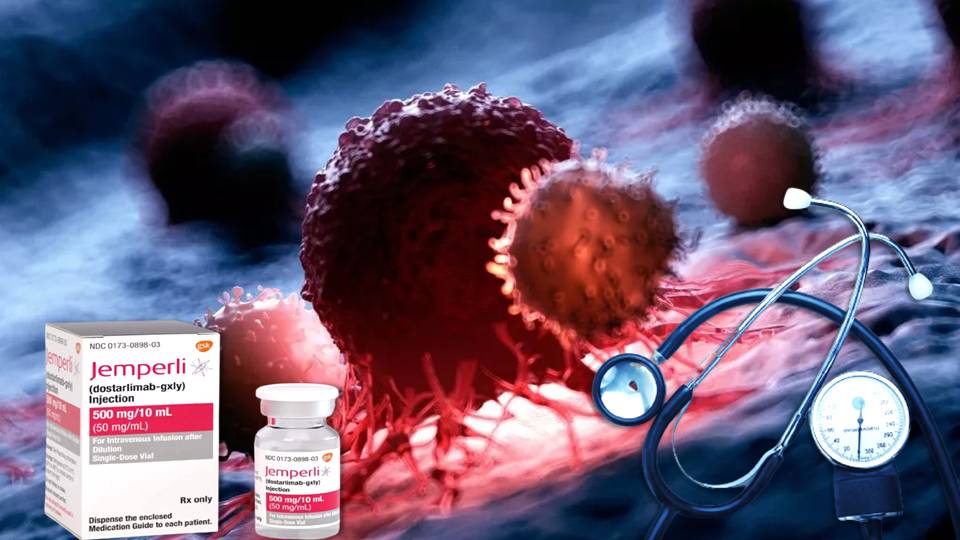
Dostarlimab, the miracle cancer cure?: A cure for cancer, a disease that affects people over a long period of time, has recently been discovered, and it has taken everyone by surprise. This is the first time in the history of the world that this has taken place.
The results of a cancer study that was just made public were “unprecedented” and “unheard-of.” At this point, all attention is focused on dostarlimab, the GlaxoSmithKline drug that was employed in the trial to achieve total remission.
According to reports, a small cancer trial that was run by a doctor in New York City has apparently achieved a result that has never been seen before: total cancer remission in each and every one of its participants.
It is true that the clinical trial, which is being led by doctors at Memorial Sloan Kettering and is being supported by the pharmaceutical company GlaxoSmithKline, has only treated a total of twelve patients. These patients all have a rare mutation and specific cancer that is in its early stages.
Despite this, the findings, which were published on Sunday in the New England Journal of Medicine as well as the New York Times, were startling enough to inspire a number of doctors to tell the publication that they believed the findings to be a first of its kind.
According to the NEJM paper and the report published in the Times, all 12 patients had rectal cancer that had not spread beyond the local area. Their tumors all had a mutation that affected cells’ ability to repair DNA damage.
Because of the drug dostarlimab, all twelve of the patients are now in complete remission. This means that none of the patients required any kind of surgery or chemotherapy, suffered no severe side effects and there is no evidence of cancer anywhere in their bodies.
While the results were encouraging, doctors interviewed by the New York Times said they needed to be replicated and expanded upon, and it was unclear whether the drug in question would be effective beyond this specific application.
Who makes Dostarlimab?
Tesaro, a biotechnology company based in Massachusetts, was responsible for the development of dostarlimab. In 2019, GlaxoSmithKline purchased Tesaro and thereby took ownership of dostarlimab.
Dostarlimab is a monoclonal antibody that was given the green light for usage as a treatment for cancer in the United States for the very first time in early 2021.
Monoclonal antibodies, such as dostarlimab, are antibodies that are manufactured in a laboratory and designed to combat particular diseases.
Over the course of the previous two years, a variety of monoclonal antibodies that can be used to treat COVID-19 have gained significantly more recognition.
Dostarlimab is a monoclonal antibody that was developed to suppress the activity of PD-1, a protein that is present in cancer cells.
Mismatch repair deficiency was found in the tumors of each of the patients who participated in the rectal cancer trial that was conducted at Memorial Sloan Kettering.
How much will it cost?
In the event that the drug is approved, the treatment will not be cost-effective. The trial itself cost $11,000 for each participant, which is equivalent to nearly N7 million for a single dose. In Nigeria, receiving treatment of this kind would not come cheap.
